Get started
The US Drug Supply Chain Security Act (DSCSA) requires the downstream supply chain (e.g. Wholesale Distributors) to verify that returned products are still valid according to the Pharmaceutical Manufacturer or Repackager who produced the products. Product Information Manager and Product Information Exchange allow direct and indirect trade partners to comply with these US DSCSA saleable returns requirements by:
- Managing product verification requests in real time across any company in the supply chain that uses a Verification Router Service (VRS), without a need for preexisting relationships between the requesting and responding parties.
- Exchanging product master data with any of their supply chain partners.
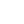
Pharmaceutical Manufacturers and Repackagers (i.e. Producers on the TraceLink Network) can sync their product master data from Master Data Exchange and serial number information from Serialized Operations Manager to Product Information Manager, which allows members of the downstream supply chain to verify serial numbers for those products and download that master data.
Members of the downstream supply chain (i.e. Consumers on the TraceLink Network) can verify serial numbers against the Producer's records in Product Information Manager and download the relevant master data for those products.
Product Information Manager uses the following industry standards to provide interoperability across the supply chain:
- VRS standards published by GS1.
- Lookup Directory standards published by the Healthcare Distribution Alliance (HDA) Task Force.
- The Global Data Synchronization Network standards published by GS1.
US DSCSA requirements
The US DSCSA, issued by the Food and Drug Administration, states:
- Wholesale Distributors must verify that returned products are still considered valid by the original Pharmaceutical Manufacturer or Repackager before these returned products can be resold.
- Pharmaceutical Manufacturers and Repackagers must respond to verification requests from Wholesale Distributors and indicate whether the product in the request is valid (i.e. can be resold).
Companies must comply with the US DSCSA saleable returns requirements for products with one or more of the following target markets:
- American Samoa
- Guam
- Northern Mariana Islands
- Puerto Rico
- United States of America
- US Minor Outlying Islands
- Virgin Islands
TraceLink and all other VRS providers establish connections with each other using X.509 certificates, which ensure that the information exchanged is secure throughout the supply chain. Each connection from TraceLink to another VRS provider has its own dedicated certificate to secure that connection. TraceLink loads and manages the x.509 certificates for customers.
Applications
Producers and Consumers describe the company's role in the supply chain when they link to a TraceLink-owned network application, such as Product Information Manager. Producers own data, which they share to the network application, and Consumers can then access this shared data.
For example, in Product Information Manager (Product Verification), Producers are Pharmaceutical Manufacturers and Repackagers who add product records and serial number information to the network application, which allows Consumers to verify products. In this example, Consumers are members of the downstream supply chain (e.g. Wholesale Distributors, 3PLs). A Pharmaceutical Manufacturer or Repackager that also acts as a Wholesale Distributor can be both a Producer and Consumer.
Customers own their instance of Product Information Exchange, however, companies might have access to only certain functions within Product Information Exchange based on whether they link to Product Information Manager as a Producer or a Consumer.
Product Information Manager is a network application, which means that TraceLink owns the app, and companies link to the app as either a Producer (i.e. a company that owns and shares data with the rest of the supply chain) or a Consumer (i.e. a company that accesses the shared data). Network apps like Product Information Manager allow companies to share data across the supply chain, even with indirect trade partners. Product Information Manager has two modules:
- Master Data Share – Provides access to product master data.
- Product Verification – Provides access to serial number verification and connects to external VRS providers.
When TraceLink customers link to Product Information Manager, they also activate Product Information Exchange. Companies own Product Information Exchange, which allows this app to access Master Data Exchange and Serialized Operations Manager data owned by the company. Product Information Exchange also gives Producers and Consumers an access point to the TraceLink-owned Product Information Manager network application.
Essentially, Product Information Manager is owned by TraceLink, and Product Information Exchange is owned by your company. These apps communicate so that your company can exchange information with indirect partners in the supply chain.
For Producers
- Master Data Exchange – Product master data entered into Master Data Exchange is synced to Product Information Manager to share with the downstream supply chain. Only the products that are synced to Product Information Manager can be verified against.
- Serialized Operations Manager – Serial number information (i.e. product code, serial number, lot number, and expiration date) that is added or updated in the Serialized Operations Manager repository as the Producer goes through the serialization process is synced to Product Information Manager. This serial number information and the item's serial number status, item status, and associated reason codes (in addition to the data rules set by the Producer) determine how Product Information Manager responds to verification requests for that serial number.
For Consumers
- Smart Event Manager – Consumers must use the Smart Event Manager Set Event REST Request to verify products against the Producer's data in Product Information Manager. This request is leveraged by both the Product Information Exchange Web UI and the Smart Inventory Tracker Mobile UI. However, Consumers must own Smart Event Manager to verify products, even if the company plans to use one of the UIs.
- Smart Inventory Tracker – Smart Inventory Tracker for Event Reporting, which integrates with Smart Event Manager, optionally allows Consumers to verify products in US markets against the Producer's data in Product Information Manager on a handheld device or smartphone.
1. Producers set up data for products that must be verified
- The Producer (i.e. a company that owns and shares data with the rest of the supply chain) adds product master data in Master Data Exchange.
- The Producer syncs that master data to Product Information Manager, through Product Information Exchange, by setting up rules that determine which data gets synced.
- A record of the products (including the Producer's GLN and the fact that TraceLink acts as the VRS for that Producer) is created in the Product Directory (i.e. Lookup Directory) and shared with external VRS providers so that members of the downstream supply chain that do not use TraceLink can verify against those products.
- The Producer syncs serial number information from Serialized Operations Manager to Product Information Manager, through Product Information Exchange, by setting rules that determine which data gets synced.
- All serial numbers currently in the Serialized Operations Manager repository that match the criteria of the rules and have a of Commissioned, Decommissioned, or Destroyed sync to Product Information Manager, through Product Information Exchange.
- As serial number information is added or updated in Serialized Operations Manager, those updates automatically sync to Product Information Manager, through Product Information Exchange.
- TraceLink regularly syncs with external VRS providers to receive records from their Lookup Directories so that TraceLink knows which VRS responds to a verification request for a specific product.
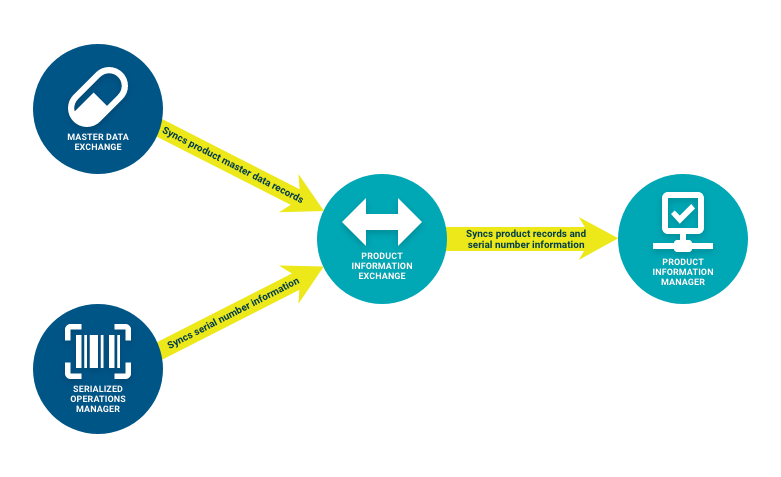
2. Consumers verify products against that data
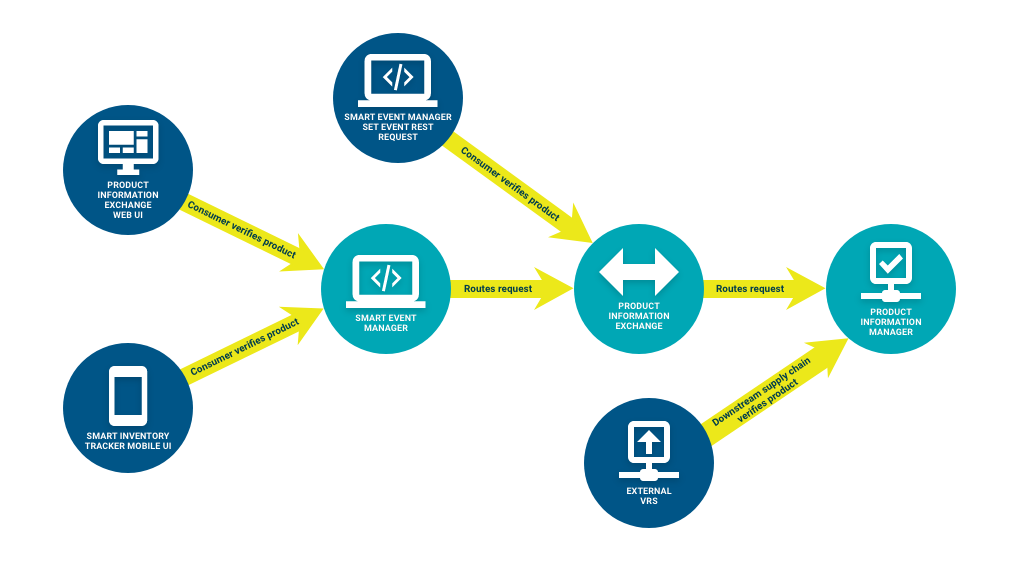
- A Consumer verifies a product with the product code, serial number, lot number, and expiration date, through one of the following ways:
- The Product Information Exchange Web UI, which uses Smart Event Manager to route the request to Product Information Manager.
- The Smart Inventory Tracker Mobile UI, which uses Smart Event Manager to route all requests with a US target market to Product Information Manager.
- The Smart Event Manager Set Event REST Request, which routes all requests with a US target market to Product Information Manager.
- An external VRS, which routes the request to Product Information Manager if the Producer that owns the product uses TraceLink.
 All verification requests that leverage Smart Event Manager always pass through Product Information Exchange before reaching Product Information Manager.
All verification requests that leverage Smart Event Manager always pass through Product Information Exchange before reaching Product Information Manager. - A Consumer downloads product master data from Product Information Manager, if necessary.
To make products available for saleable returns verification
- Add product master data through one of the following:
 See a quick reference for required fields only.
See a quick reference for required fields only. - Set data rules to sync products to Product Information Manager through one of the following:
A record of the products (including the Producer's GLN and the fact that TraceLink acts as the VRS for that Producer) is created in the Product Directory (i.e. Lookup Directory) and shared with external VRS providers so that members of the downstream supply chain that do not use TraceLink can verify against those products.
- Set data rules to sync serial number information to Product Information Manager through one of the following:
All serial numbers currently in the Serialized Operations Manager repository that match the criteria of the rules and have a of Commissioned, Decommissioned, or Destroyed sync to Product Information Manager, through Product Information Exchange.
- Set custom verification response rules to specify the amount of information returned in verification responses through one of the following:
- As serial number information is added or updated in Serialized Operations Manager, those updates sync to Product Information Manager, through Product Information Exchange.
Consumers can then verify against these products or download the product master data they need. The verification requests can come from Consumers on the TraceLink Network or from an external VRS provider on behalf of the downstream supply chain member.
After products are available for verification
Producers can:
- Check the Product Directory to make sure the records have been created successfully in Product Information Manager through the:
- Verify their own products to ensure the information in Product Information Manager is correct through one of the following:
- Download a history of all verifications performed on their products to see who is verifying and which products they are verifying against through one of the following:
Consumers can send verification requests with the product code, serial number, lot number, and expiration date through the:
- Product Information Exchange Web UI
- Smart Inventory Tracker Mobile UI
 To verify product through Smart Inventory Tracker, Consumers must register devices though the Administration Web UI or the Device Manager REST Messages.
To verify product through Smart Inventory Tracker, Consumers must register devices though the Administration Web UI or the Device Manager REST Messages. - Smart Event Manager Set Event REST Request
Both UIs leverage the Smart Event Manager Set Event REST Request, which is sent to Product Information Exchange for all US target markets. Product Information Exchange then routes the verification request to Product Information Manager, which responds to the request for all products shared through TraceLink or sends the request to an external VRS.
Consumers can also download product master data from Producers through the Product Information Manager REST Messages.
The Product Information Exchange Web UI supports Zebra DS2200 scanners for verifying products. All barcodes must contain the product (01), serial number (21), lot number (10), and expiration date (17).
- Commercially available warehouse scanning devices leveraging an Android operating system (versions 11–13). Currently, the TraceLink-validated devices are:
- Zebra MC3300, MC3300x, MC9300, MC9400, TC70x, TC72, TC77, TC8300, TC51, and TC52.
- Honeywell CT60, CN80, and CK65.
- Commercially available smartphones leveraging an Android operating system (versions 11–13). Currently, the TraceLink-validated devices are:
Google Pixel 2 and Pixel 7
Samsung Galaxy S9 and Galaxy Xcover
 Devices running on Android 8, 8.1, 9.0, and 10 OS will no longer be supported with feature enhancements and bug fixes in the SIT app after March 2024.
Devices running on Android 8, 8.1, 9.0, and 10 OS will no longer be supported with feature enhancements and bug fixes in the SIT app after March 2024.
Yes. If Smart Inventory Tracker is installed on the device with an Android Packaging Kit (APK) that supports communication through a proxy server, then all communication from Smart Inventory Tracker to the TraceLink Track & Trace Services is routed through a proxy server using HTTP protocol. Download the Smart Inventory Tracker Installation Qualification Document for more information.
Track & Trace Services
TraceLink supports the following browsers:
- Google Chrome: The most recent stable version.
- Mozilla Firefox: The most recent stable version.
- Microsoft Edge: The most recent stable version; tied to the Microsoft Support Lifecycle.