South Korea Compliance Reporting
South Korea Compliance Overview
The South Korea Compliance application (app) helps Pharmaceutical Manufacturers and Wholesale Distributors comply with the South Korean government's serialization and reporting requirements.
Use the South Korea Compliance app to search and view various South Korea Compliance reports that have been sent to the Korean Pharmaceutical Information Service (KPIS). See Available Functions below for details.
South Korea Compliance operates with either of the following:
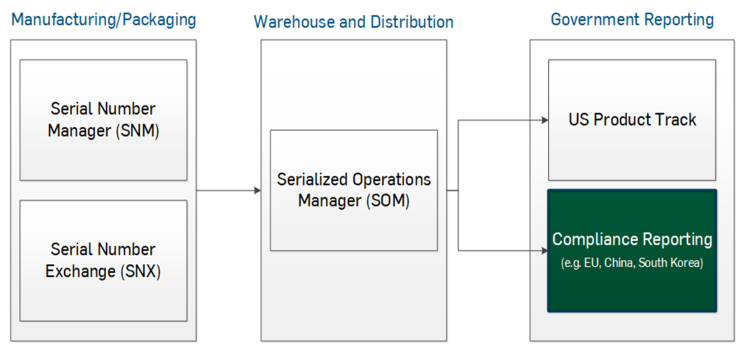
- TraceLink's Serialized Operations Manager: Manages and reports on serial numbers, their corresponding products, and containers. TraceLink integrates serialized product operations with warehouse operations and stores serialized product information in Serialized Operations Manager.
- External Serialization Manager (ESM): TraceLink integrates with external systems of record (SOR). Companies using external systems to generate and manage serial numbers use ESM for transferring external serial numbers and Serialized Operations Manager for sending those reports through TraceLink.
![]() South Korea provides an Enterprise Service Bus (ESB) adapter tool, which TraceLink uses to automatically submit reports to the Korea Pharmaceutical Information Service (KPIS).
South Korea provides an Enterprise Service Bus (ESB) adapter tool, which TraceLink uses to automatically submit reports to the Korea Pharmaceutical Information Service (KPIS).
The South Korea Government introduced the Pharmaceutical Affair Act, Article 45, which outlines serialization requirements for South Korea. The law in it's current state places compliance responsibility on Pharmaceutical Manufacturers and Wholesale Distributors. The law addresses:
- Serialization
- Aggregation
- Reporting
Getting Started in South Korea Compliance
Have a user with administrator access configure workflows to generate reports.
![]() See Configure Event Workflows and Event Triggers topics for configuration details.
See Configure Event Workflows and Event Triggers topics for configuration details.
Some optional fields might be mandatory based on South Korea's regulatory requirements. Download the Quick Reference Guide to view their specific required fields.
Available Functions
The table on the Search Compliance Reports screen displays the Korea Pharmaceutical Information Service (KPIS) reports that TraceLink created. Change the reports displayed by altering any of the available search criteria.
The View Sales Shipment Report Details screen displays the report information, supplier information, receiver information, and shipment information associated with the specific Sales Shipment Reports submitted to KPIS. Aggregation information can be viewed in the View Serialized Item Aggregation Details screen.
The View Sales Shipment Notice Details screen displays the report information, supplier information, receiver information, and shipment information associated with the specific Sales Shipment Notice submitted to KPIS. Aggregation information can be viewed in the View Serialized Item Aggregation Details screen.
The View Return Receipt Report Details screen displays the report information, supplier information, receiver information, and shipment information associated with the specific Return Receipt Report submitted to KPIS. Aggregation information can be viewed in the View Serialized Item Aggregation Details screen.
The View Return Receipt Notice Details screen displays the report information, supplier information, receiver information, and shipment information associated with the specific Return Receipt Notice submitted to KPIS. Aggregation information can be viewed in the View Serialized Item Aggregation Details screen.
The View Cancel Shipment Report Details screen displays the report information, supplier information, receiver information, and shipment information associated with the specific Cancel Shipment Report submitted to KPIS. Aggregation information can be viewed in the View Serialized Item Aggregation Details screen.
The View Decommission Report Details screen displays the report information, supplier information, and shipment information associated with the specific Decommission Report submitted to KPIS. Aggregation information can be viewed in the View Serialized Item Aggregation Details screen.
The View Correct Shipment Report Details screen displays the report information, supplier information, and shipment information associated with the specific Correct Shipment Notice submitted to KPIS. Aggregation information can be viewed in the View Serialized Item Aggregation Details screen.