How Product Track Manages Transaction Histories
As product moves through the pharmaceutical supply chain from manufacturer to wholesaler to pharmacy, TraceLink maintains a transaction history showing each change of ownership.
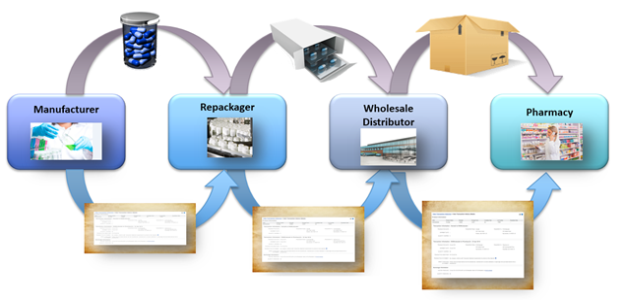
The supply chain can include many partners such as contract manufacturers, repackagers, and secondary wholesalers. TraceLink’s visibility into the supply chain protects against tainted or counterfeit product slipping into the pharmaceutical market.
For the lot level phase, TraceLink generates compliance documentation from the Advance Ship Notice (ASN) sent by the supplier. For paper-based interactions, a receiving associate enters the data manually and Product Track generates the transaction information. If there are issues with a shipment - for example, the quantity sent does not match the quantity in the ASN - the shipping and receiving teams use Product Track to resolve exceptions and maintain compliance.
The compliance documentation is stored as “transaction histories.” The following diagram describes the contents of a transaction history.
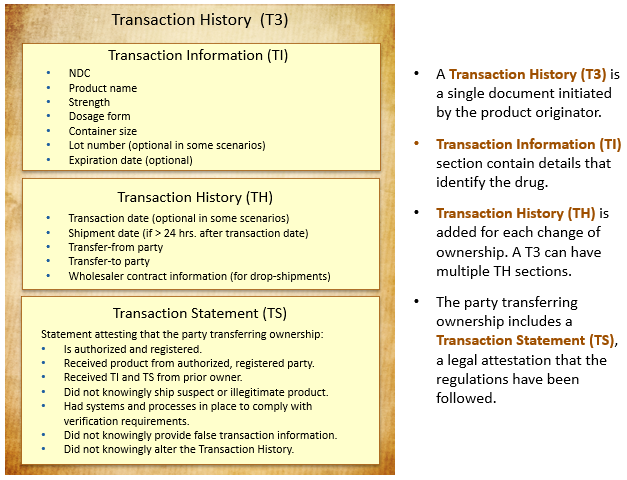
For the final regulation, the transaction history portion of the T3 is deprecated. Instead, supply chain partners exchange T2 information (Transaction Information and the Transaction Statement) and serialization information electronically.
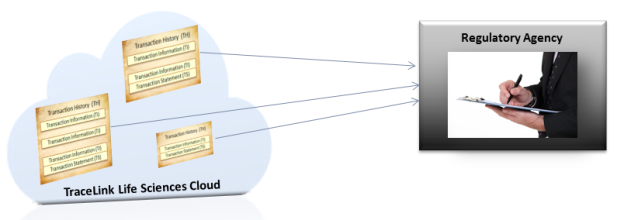
If a regulatory agency requests compliance information about a drug, companies in the TraceLink network use Product Track to search for a particular product and lot number to provide the required compliance and serialization data to the agency.